F Energy Level
At the fourth and higher levels there are seven f orbitals in addition to the 4s 4p and 4d orbitals. F fracmv2r1 Also the electrostatic force of attraction between the nucleus of charge Ze and the electron is - e will be.

Pin By Jaclyn Devincentis On Physics Teaching Chemistry Energy Level F Names
These distances known as orbitals are of different shapes denoted by a letter s p d f.

F energy level. Emission originating from a certain energy transitions from the ground state to the low-spin state level can occur when the energy gap to the next lower do not change the spin of the electrons and are thus level is more than four or five times the maximum f article in press 452 ps. The energy level of the bonding orbitals is lower and the energy level of the antibonding orbitals is higher. Sharp-s Principle-p Diffuse-d Fundamental-f In an atom there are some combinations of these orbitals.
They have even more complicated shapes. Different orbits in which electrons revolve are known as stationary states or energy levels. The electric field E is interfered with two processes which are the electric current density j and the temperature gradient T The coefficients come from the Ohms law and the Seebeck effect.
Each energy level beginning with n4 contains f orbitals of which there will always be seven having identical energy. W Fs Where W Work F Constant Force N S Distance moved in the direction of the force m SI Unit for work is joules J One joule is defined as the work done when a force of 1N moves an object through a. In order to find the energy of the photon that was absorbed or emitted you always take the higher energy level and subtract from it the lower energy level.
The Fermi energy is a concept in quantum mechanics usually refers to the energy difference between the highest and lowest occupied single-particle states in a quantum system of non-interacting fermions at absolute zero temperature. So in this case we would take -6eV and subtract from it -10eV which tells us that it would take a four eV photon to bump an electron up to that energy level and the electron would emit a four eV photon if it dropped back down from. It is the energy level which is occupied by the highest electron orbital at 0 Kelvin absolute zero temperature and a parameter of the Fermi-Dirac distribution.
Each type of orbital has its own characteristic shape. The Fermi Level with Fermi energy Ef is the surface of this sea where electrons will not have enough energy to rise above the surface. The fourth and higher levels also have an f sublevel containing seven f orbitals which can hold a maximum of 14 electrons.
Therefore s p d f energy levels have a maximum of 2 6 10 14 electrons respectively. 2 in the s orbital 6 in the three p orbitals 10 in the five d orbitals and 14 in the seven f orbitals. F frac14πε₀ fracZeer22 We know that K frac14πε₀ F K fracZeer2 KfracZe2r2 Now equating eq 1 2 we get.
Thus the fourth level can hold up to 32 electrons. The fourth and higher levels also have an f sublevel containing seven f orbitals which can hold a maximum of 14 electrons. There are four types of orbitals- s p d and f.
Aufbau principle and electron configuration German scientist Aufbau expresses the building up principle for the electron configuration process in different electronic orbitals of atoms. Journal of solid state chemistry 178 2005. Considering this how many f orbitals exist in one energy level of an atom.
In addition the third and subsequent energy levels each contain five D-Orbitals the fourth and subsequent energy levels contain seven F-Orbitals and so on. For example in the 4th energy level 4s is the lowest energy closest to the nucleus then 4p. S P and D Orbitals do not all have the same energy.
Counting the 4s 4p and 4d orbitals this makes a total of 16 orbitals in the fourth level. Thus the fourth level can hold up to 32 electrons. For the bond in the molecule to be stable the covalent bonding electrons occupy the lower energy bonding orbital which may be signified by such symbols as.
Bohrs Explanation for Energy Level. These integers are also known as the principal quantum numbers. S p d and f orbitals are available at all higher energy levels as well.
Work is done when a force moves the object in the direction of the force and is given by the product of the force and the distance moved in the direction. The energy level is a quantity of space or regions wherever electrons will probably be present. There are 7 f orbitals in each energy level.
In the course of solving this equation one generates quantum numbers that determine the characteristics of the electrons in an atom. The field can be written as 11-2 T j 111 where is the electrical conductivity and is. For an f orbital l 3 and the possible values of ml are 3 2 1 0 1 2 and 3 so there are seven f orbitals in each energy level n 4.
The value of the Fermi level at absolute zero temperature 27315 C is known as the Fermi energy. There are four types of orbitals that you should be familiar with s p d and f sharp principle diffuse and fundamental. In the n1 shell you only find s orbitals in the n2 shell you have s and p orbitals in the n3 shell you have s p and d orbitals and in the n4 up shells you find all four types of orbitals.
2 in the s orbital 6 in the three p orbitals 10 in the five d orbitals and 14 in the seven f orbitals. Within each shell of an atom there are some combinations of orbitals. Number of Electrons in each Level.
22 State the relative energies of spd f orbitals in an energy level SL IB Chemistry - YouTube. The pattern you speak of that tells us the number of orbitals of each type results from solving of Schroedingers equation. These stationary states energy level for an electron are numbered as n 1 2 3.

Energy Level Wikiwand Energy Level Energy Quantum Mechanics

Chemistry Quantum Numbers Chemistry Classroom Teaching Chemistry Chemistry Lessons

Periodic Table Database Chemogenesis Periodic Table Internet Database Surprising Facts

What Do S P D And F Mean In Chemistry And Why Do You Need Them Chemistry High School Chemistry Electron Configuration

The Atom Chemistry Is My Jam Chemistry Chemistry Classroom Electron Configuration

The Atom Chemistry Is My Jam Electron Configuration Chemistry Chemistry Help

F Block Elements Study Chemistry Electron Configuration Name Symbols

Pin By Clemen On Libros Chemistry Teaching Chemistry Study Materials

The Atom Chemistry Is My Jam Chemistry Energy Level Low Energy Level

Absorption And Emission Of The Photon By An Electron In The Hydrogen Atom Even Light Is F Physics And Mathematics Medical Laboratory Science Quantum Mechanics

D Orbitals Chemistry Organic Chemistry Chemistry Experiments

What Is A Polar Covalent Bond Tutor Pace Covalent Bonding Tutor Energy Level

The Atom Chemistry Is My Jam Chemistry Energy Level Low Energy Level
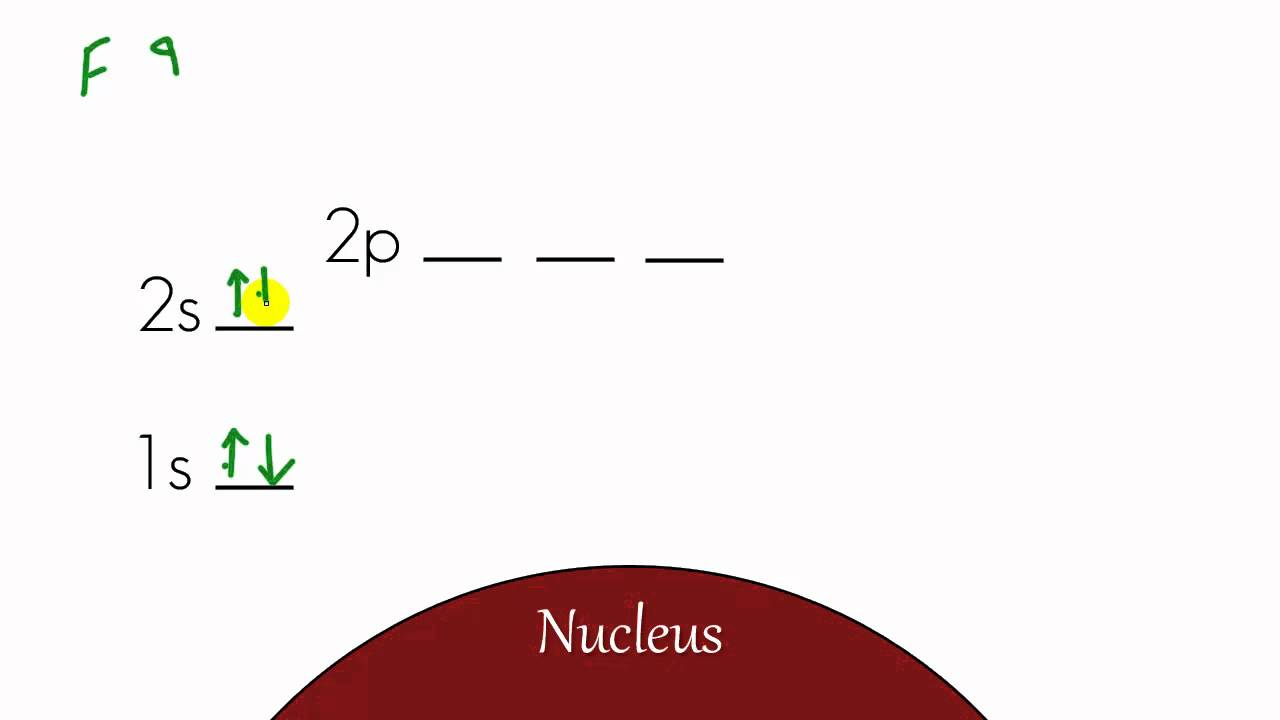
Chemistry Lesson 12 Energy Level Diagram And Electron Configuration Youtube Chemistry Lessons Electron Configuration Chemistry
Posting Komentar untuk "F Energy Level"